I've been substituting KCl for NaCl for about a year, with excellent blood pressure results:
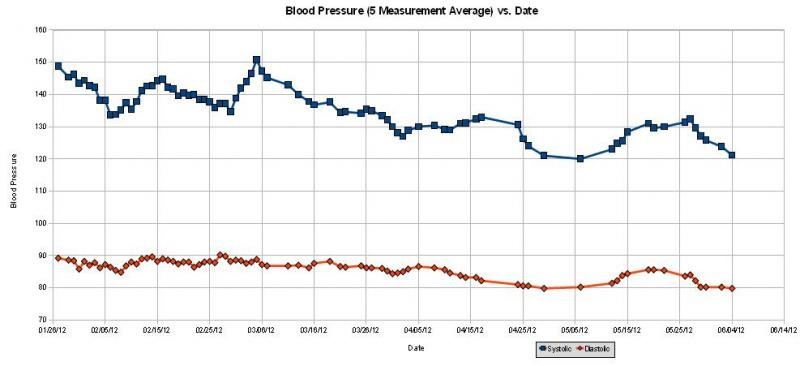
Although some people don't like the taste of KCl, I find it to be almost identical in taste to normal salt (and see below). I even replace NaCl with KCl in bacon, by soaking the bacon to remove the regular salt, then adding KCl.
So, I'm considering brining our turkey in a solution of KCl. To hedge my bets, I'll probably make the solution more dilute that normal. Has anyone tried this? Any thoughts?
Concerning the taste of KCl, consider this story. When my daughter was visiting she said she didn't like salt substitutes. I put a small pile of salt on each of two plates, and had her do a taste comparison. "Oh, yuck, that second one is the KCl, it tastes terrible!" she said.
What she didn't know was that both piles were regular NaCl table salt. IOW, we can't always trust our senses.
Thanks,
Al

Although some people don't like the taste of KCl, I find it to be almost identical in taste to normal salt (and see below). I even replace NaCl with KCl in bacon, by soaking the bacon to remove the regular salt, then adding KCl.
So, I'm considering brining our turkey in a solution of KCl. To hedge my bets, I'll probably make the solution more dilute that normal. Has anyone tried this? Any thoughts?
Concerning the taste of KCl, consider this story. When my daughter was visiting she said she didn't like salt substitutes. I put a small pile of salt on each of two plates, and had her do a taste comparison. "Oh, yuck, that second one is the KCl, it tastes terrible!" she said.
What she didn't know was that both piles were regular NaCl table salt. IOW, we can't always trust our senses.
Thanks,
Al
